Home >> Blog >>Ionization Techniques - The Evolution and How MALDI and ESI MS/MS Eases in-depth Analysis of Glycans
Ionization Techniques - The Evolution and How MALDI and ESI MS/MS Eases in-depth Analysis of Glycans
January 17, 2022
Glycosylation is covalent attachment of a carbohydrate to form a glycoconjugate. It is the most abundant and complex post-translational modification found in proteins, where the glycans play a critical role in the stability, bioactivity, and immunogenicity of the protein. Therefore, knowing the site of glycosylation and revealing the detailed structural features of glycans are very important for studying the potential therapeutic importance of a glycoprotein.
Mass spectrometry (MS), more specifically, tandem mass spectrometry (MS/MS) appeared as the gold-standard method for glycan characterization because of sensitivity, specificity, and speed. For mass spectrometry-based analysis, the neutral molecules must convert to ions. The sample of interest is first subjected to an ionization source in the mass spectrometer, where they are ionized (acquires positive or negative charge). The ions then travel through an electromagnetic field in the mass analyzer, deflect based on their m/z ratio, and are recorded in the different parts of the detector. Therefore, ionization techniques play a vital role in the qualitative and quantitative analysis of the glycans. There are two types of ionization methods available – hard ionization, and soft ionization.
-
Hard Ionization: It produces a large amount of internal energy in a sample to ionize it. This technique typically generates a large number of low mass fragments.
-
1. Electron Ionization (EI): It was one of the pioneers of ionization techniques, where a high-energy electron beam is used to bombard a sample to produce extensive fragment ions. This technique is mostly used in GC-MS.
Advantages
High internal energy leads to extensive fragmentation that helps in detailed structural elucidation
Disadvantages
Not ideal for the characterization of large glycoproteins
Extensive fragment generation may lead to possible ambiguous identification
More fragment ions make the spectrum interpretation difficult
Only positive ions are formed
Soft Ionization: The soft ionization techniques use less energy for ionizing a sample and thus produce a higher amount of high mass fragments. This method is suitable for biological samples with large molecular mass (such as polypeptide, oligosaccharides, etc.), because it does not fragment a macromolecule into tiny charged particles. Instead, it ionizes the macromolecule into small droplets.
The modern mass spectrometry-based glycan characterization strategies employ soft ionization techniques. Some of those techniques are:
1. Fast Atom Bombardment (FAB): It was the first popular ionization technique between the 80s to early 90s that facilitated the ionization of macromolecules. In this technique, the sample is mixed with a matrix (glycerol or 3-nitrobenzoic acid), placed on the surface, and subjected to sequential bombarding using high-energy atoms of inert gas (Xenon and Argon) to produce the ions.
Advantages
Ideal for macromolecules such as polypeptides, oligosaccharide analysis
Large mass fragments are generated
Allows ionizing polar, thermally, and energetically labile samples
Produces less complicated spectra with singly charged ions
Disadvantages
Crucial characterization using small fragments is difficult
Lower sensitivity
Dependency on a matrix
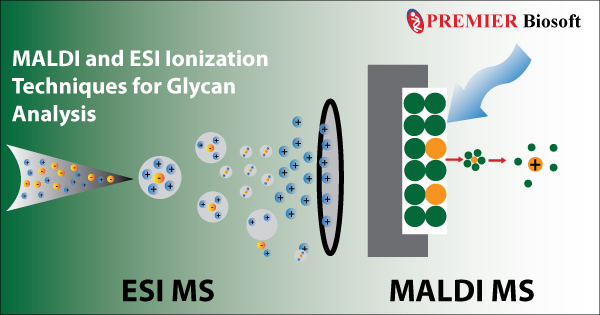
2. Matrix Assisted Laser Desorption Ionization (MALDI): It is one of the most popular soft ionization techniques used in modern mass spectrometry-based glycan analysis. As the name implies, this technique mixes the sample in a matrix, which is further bombarded using a laser. The matrix absorbs the laser radiation and transfers a proton to the sample converting the neutral sample to an ion. In addition to the conventional fragment-based mass spectrometric analysis, MALDI is extensively used in imaging mass spectrometry.
Advantages
Samples with large mass can be analyzed
Ideal for large molecules such as polypeptides, oligosaccharides, DNA, etc.
Generates less complicated mass spectra with singly charged ions
Disadvantages
Crucial characterization using small fragments is difficult
Possible ion suppression – the matrix ions suppress the molecular ions
Possibility of photodegradation by laser desorption/ionization
Not recommended for acidic monosaccharides (sialic acids, uronic acids), substituents (sulfate, phosphate), and fucose, as the technique dissociates the labile glycosidic bonds and the abundances of these monosaccharide residues are underestimated.
3. Electrospray Ionization (ESI): Previously developed soft ionization techniques such as FAB, and MALDI undoubtedly facilitated the ionization of small glycoproteins, however, the large glycoproteins were still out of reach. In 1984, Masamichi Yamashita and John Fenn developed the revolutionary Electrospray Ionization (ESI) method, where the sample of interest mixed in a liquid matrix is dispersed into fine aerosols using a high-voltage electrospray. This method allowed the ionization of large glycoproteins by overcoming the propensity of the large molecules to fragment themselves when ionized.
This technique produces very few fragments, insufficient for detailed structural elucidation. However, coupling along with tandem mass spectrometry (ESI-MS/MS) overcomes this challenge. In addition to this, ESI allows analysis of intact native glycans without dissociating the fragile acidic groups (sialic acids, uronic acids), and thus have an edge over MALDI.
Advantages
Ideal for large mass samples (such as polypeptides, oligosaccharides, DNA, etc.)
The ESI generates ions with multiple charges, which allows the analysis of large molecules on analyzers with limited m/z ranges
Recommended for glycans with acidic monosaccharides
No matrix is needed
Highly compatible with liquid chromatography
Disadvantages
Insufficient number of fragments for detailed characterization of the sample (which can be overcome by coupling along a mass spectrometer)
Mass spectra with multi-charged ions are too complex for manual interpretation and annotation
The current generation mass spectrometry-based analytical platforms, MALDI-MS/MS, ESI-MS/MS adequately ionize glycan samples and generate necessary fragments for comprehensive characterization. A mass spectrum displays the relative abundance of the ions against their corresponding m/z ratio. The mass spectra of single protonated, deprotonated, or other singly charged ions are easy to interpret, however, appear tedious and time-consuming. ESI also generates multi-charged ions that make a mass spectrum too complicated to annotate the fragment ions manually. Informatic tools can help with the fast and accurate interpretation of complex mass spectra. SimGlycan® is a software tool that supports all the above-discussed ionization techniques, automatically annotates the MS/MS fragment ions, and reports the candidate glycan structures in a ranked manner. For more information, please schedule a meeting with us.
| Comment | Share |
|


